The COVID-19 Antigen Test is an in vitro immunochromatographic method for the qualitative detection of SARS-CoV-2 nucleocapsid protein antigens from the saliva of individuals suspected of COVID-19.
Saliva-based tests are especially convenient compared to nasal or oropharyngeal swabs making it easier for people to self-test.
Specimen types: saliva
Testing time: 10-15 minutes

How to Use 2019-nCoV Ag Rapid Test Kit
| 1 ➜ | 2 ➜ | 3 ➜ | 4 ➜ | 5 ➜ |
|---|---|---|---|---|
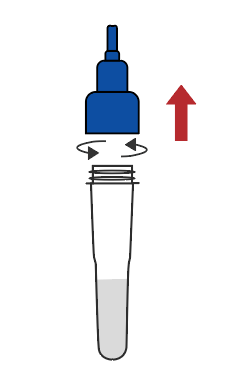 Remove the cap of the extraction buffer | 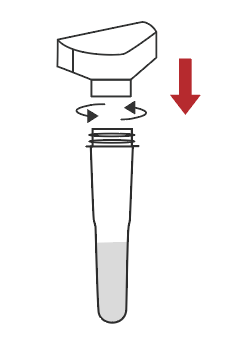 Connect the saliva collector to the extraction buffer tube |  Cough deeply three times, and spit out saliva from the back of the oropharynx to the collector, saliva volume is up to the line | 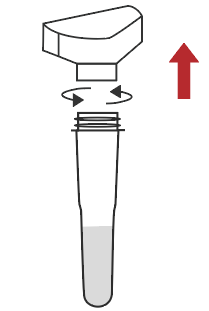 Remove the saliva collector | 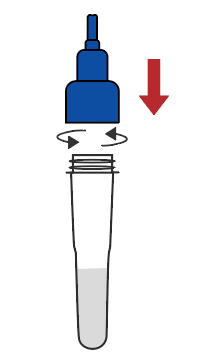 Return the cap |
| 6 ➜ | 7 ➜ | 8 ➜ | 9 ➜ | 10 |
|---|---|---|---|---|
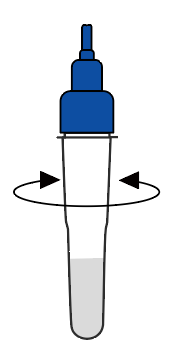 Shake the tube at least 1 min to mix the saliva and extraction buffer thoroughly | 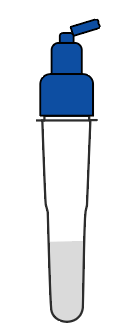 Break the pin on the top | 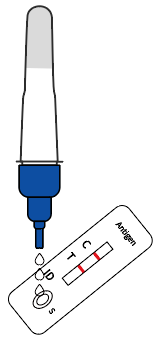 Add 3~4 drops of the processed sample solution (about 80 μL) into the sample well | 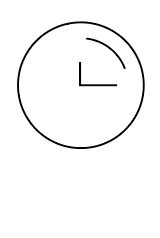 Wait 10-15 minutes | 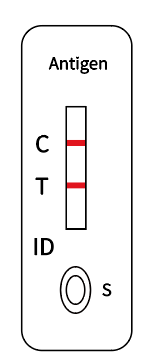 View the results |
NOTE:
- Clean your hands before touching the tool.
- Do not eat for 30 minutes before collection.
- Rinse your mouth with water 30 minutes before saliva collection to clean up the residue.
- Pressing your tongue against the bases of your upper and lower teeth will help you spit out a sufficient amount of saliva in a short amount of time.
- The amount of saliva collected must reach the calibration line.
- Used collection tools should be disposed of in the dedicated clinical waste bin.
Product Details
The self-test COVID-19 Ag rapid test kit is to identify SARS-CoV-2 nucleocapsid antigen that is generally detectable in upper respiratory samples during the acute phase of infection.
The detection area (T) on the plain film is pre-coated with an anti-2019-nCoV monoclonal antibody, and finally, a red reaction line is formed in the T area. If the sample does not contain the 2019-nCoV antigen, a red reaction line cannot be formed in the T zone.
- Sensitivity: 96.15% (100/104), confirmed positive cases compared to the nucleic acid amplification test.
- Specificity: 99.78% (448/449), confirmed negative cases compared to the nucleic acid amplification test.
- Cross-reactivity: There is no cross-reactivity with influenza A virus, influenza B virus, adenovirus, Coxsackie virus, ECHO virus, and enterovirus; no cross-reactivity with Chlamydia pneumoniae, Mycoplasma pneumoniae, Chlamydia psittaci, and Chlamydia trachomatis; no cross-reactivity with Acinetobacter baumannii, Bordetella pertussis, Candida albicans, Escherichia coli, Haemophilus influenzae and Neisseria gonorrhea.
Application:
For suspicious patients with symptoms, mild symptoms, or even without symptoms, also for testing people with close contact with infected patients and people under quarantine control.
 Oropharyngeal Swabs, Virus Transport Media, Saliva Collector_Huachenyang
Oropharyngeal Swabs, Virus Transport Media, Saliva Collector_Huachenyang






